Was it an earthquake, or had a trainload of nitroglycerine exploded? In the early evening of 2 May 1878, people around the city of Minneapolis ran from their houses as windows were blown out and blocks of granite were hurled across eight city blocks. The city was famous for its mills, but few people guessed the cause of the explosion: flour dust.
Collision theory can explain the devastation of that night. Flour dust in the air of the world’s biggest mill created a huge surface area on which reactions could occur. A single spark – perhaps from two dry millstones grinding together – supplied the activation energy to ignite nearby flour dust in oxygen that triggered the explosion.
What students need to know
Most learners by age 14 understand that chemical reactions produce new substances; that a large change in temperature, like an explosion, can be a sign of a chemical reaction; and that particles gain energy when you heat them. At 14–16, students need to know:
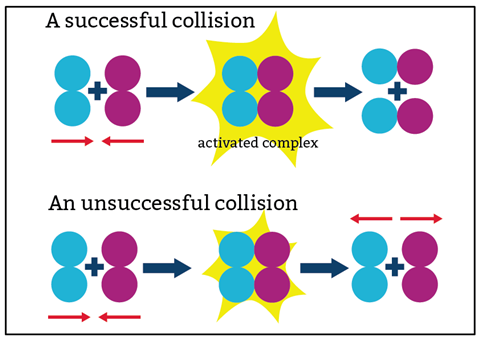
- For a chemical reaction to occur, particles must collide.
- For a collision to be successful, particles must collide with a minimum level of energy – the activation energy.
- The rate of reaction depends on the frequency of successful collisions, which is affected by temperature, pressure, concentration, surface area and whether there is a catalyst.
- A reaction profile follows the energy levels of reactants as a reaction progresses to form products.
What you need to know
At post-16, the idea that a single collision converts reactants into products is replaced by mechanisms that often occur in multiple steps. Simple reaction profiles have a single peak that represents the activation energy. However, multi-step mechanisms have several peaks, the largest of which represents the slow, rate-determining step.
At post-16, the idea that a single collision converts reactants into products is replaced by mechanisms that often occur in multiple steps. Simple reaction profiles have a single peak that represents the activation energy. However, multi-step mechanisms have several peaks, the largest of which represents the slow, rate-determining step.
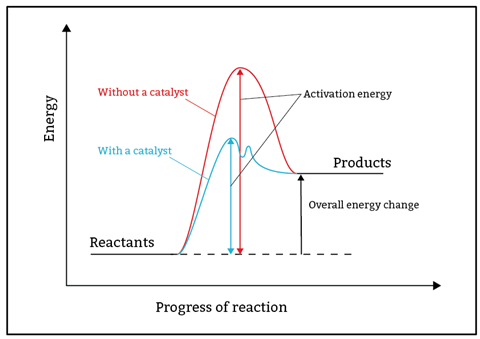
Prepare learners for these mechanisms by modelling the alternative pathway provided by catalysts with reaction profiles that have multiple peaks. The profiles will serve as a useful reference point when learning progresses to mechanisms.
Common misconceptions
The concept that particles collide and react often comes readily to learners who already know that particles vibrate and move. What can follow is the misconception that all collisions result in a reaction. Teachers cannot repeat the phrase, ‘Not all collisions are successful’, too often.
Another misconception is that an increased rate of reaction results in more product – it doesn’t, but it can affect how fast the product is formed. This misconception may seem reasonable when learners watch how quickly a gas syringe or measuring cylinder fills with gas at higher reactant concentrations, so be clear here. Take care when preparing experiments and choose concentration ranges that will ensure all reactions reach completion in the time available. Asking students to analyse experimental graphs will reinforce the idea that the total volume of product is unchanged regardless of how quickly the reaction occurs.
Suggestions for your teaching
Learners need to understand collision theory on the three levels of Johnstone’s triangle: the macroscopic observations made in experiments, the submicroscopic explanations of what is happening at the particle level and symbolic representations, including graphs.
Learners need to understand collision theory on the three levels of Johnstone’s triangle (rsc.li/43u7sJB): the macroscopic observations made in experiments, the submicroscopic explanations of what is happening at the particle level and symbolic representations, including graphs.
However, trying to gain mastery of all three levels at once can slow down the learning process. As you progress through the factors that affect the rate of reaction, try to include just two sides of Johnstone’s triangle at any given time.
Concentration matters
Start the topic by explaining the necessity of successful collisions and ask learners to predict the effect of increased concentration. They can test out their ideas by investigating the reaction between acid and metal, or the disappearing cross experiment.
Start the topic by explaining the necessity of successful collisions and ask learners to predict the effect of increased concentration. They can test out their ideas by investigating the reaction between acid and metal, or the disappearing cross experiment (rsc.li/4j4LKBv).
An analogy can help explain what is going on. Imagine children in a playground. They might be somewhat accident prone, but collisions do not always result in scuffed knees. Increasing concentration is like adding more children to the playground. Collisions will occur more frequently, so there will be more scuffed knees. In the same way, a higher number of reactant particles in the same volume will mean more frequent collisions, so the rate of successful collisions increases.
Turn up the heat
Many learners may correctly predict the effect of increased temperature. However, they might explain it in the same way as increasing concentration, reasoning that both increase the frequency of collisions so both increase the rate of reaction.
Explain that an increase in temperature means particles gain energy. Although collisions will occur more frequently, they also occur with higher average energy, so a larger proportion are successful.
Increasing the temperature of a reaction is like children in a playground going from walking to running. With higher average energy levels (running), there will be more collisions. And when those collisions occur, they are more likely to result in scuffed knees.
It is worth noting that even if most of the children are running about, not all children will be doing so. Not all reactant particles have high energy, even at high temperatures. Learners can visualise the distribution of energies in reactant particles with an online simulation. Challenge them to try to follow the movement of one particle at a time.
It is worth noting that even if most of the children are running about, not all children will be doing so. Not all reactant particles have high energy, even at high temperatures. Learners can visualise the distribution of energies in reactant particles with an online simulation, like this one from Falstad: bit.ly/43egcDi. Challenge them to try to follow the movement of one particle at a time.
Move the story on by observing the breakdown of hydrogen peroxide when you add some washing-up liquid. Learners will be unimpressed – until you add different catalysts.
Move the story on by observing the breakdown of hydrogen peroxide (rsc.li/3SHOolU) when you add some washing-up liquid. Learners will be unimpressed – until you add different catalysts.
Look under the surface
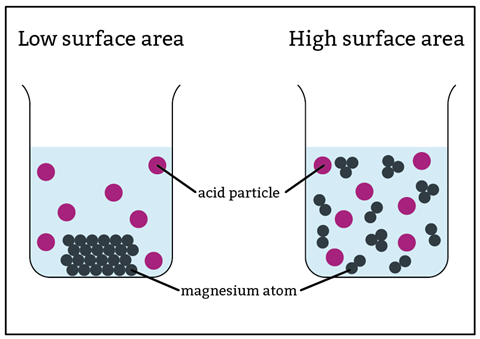
The effect of surface area on rate of reaction is not always intuitive. Smaller lumps react more quickly because more reactant particles are on the surface where they can take part in collisions.
To understand this, you need to convince learners that smaller lumps do indeed have a larger surface area. Ask students to cut a playdough cube into eight smaller cubes. They will easily see the increase in surface area.
A classic lump versus powder experiment shows this effectively, but a results table rarely leaves a lasting impression. Igniting a fine powder is much more memorable! Show learners the dramatic effect that a large surface area can have with a cornflour ‘bomb’ demonstration that mimics a mill explosion but on a safe scale.
A classic lump versus powder experiment shows this effectively, but a results table rarely leaves a lasting impression. Igniting a fine powder is much more memorable! Show learners the dramatic effect that a large surface area can have with a cornflour ‘bomb’ demonstration that mimics a mill explosion but on a safe scale (rsc.li/3H5imhf).
Kate Comisso is a chemistry teacher turned science writer based in the Scottish Highlands
Resources for your classroom
- When learners feel confident, use this practical video resource to link experimental work with both graphs and an explanation of what is happening at the particle level – the three sides of Johnstone’s triangle.
- Reinforce learning about reaction profiles and catalysts with these scaffolded worksheets for 14–16s featuring the explosive defences of the bombardier beetle.
- Strengthen understanding of energy distributions and scaffold progression to post-16 learning with this resource on the Maxwell–Boltzmann distribution.
- Link lessons on catalysts to careers with Misbah’s video job profile. She is a senior principal scientist who uses computational chemistry to help remove harmful pollutants from road-using vehicles.
Resources for your classroom
- When learners feel confident, use this practical video resource to link experimental work with both graphs and an explanation of what is happening at the particle level – the three sides of Johnstone’s triangle: rsc.li/43DeGvW
- Reinforce learning about reaction profiles and catalysts with these scaffolded worksheets for 14–16 featuring the explosive defences of the bombardier beetle: https://rsc.li/45k11et
- Strengthen understanding of energy distributions and scaffold progression to post-16 learning with this resource on the Maxwell–Boltzmann distribution: rsc.li/4dJLWFz
- Link lessons on catalysts to careers with Misbah’s video job profile. She is a senior principal scientist who uses computational chemistry to help remove harmful pollutants from road-using vehicles: rsc.li/43F9g3z

